A new CBD gel is being tested on patients in New Zealand with a specific form of epilepsy – ZYN002 is a non-THC topical gel from Zynerba Pharmaceuticals.
“The NZ government’s approval of this trial to assess a new cannabis gel for treatment of seizures, is an important step forward in the NZ government’s recognition of the potential of cannabis and various cannabinoids for medical purposes,” said Sue Grey, a New Zealand lawyer. Grey has recently been flooded with requests from medical marijuana patients in need of legal defense.
It’s not the norm, as the availability of cannabinoids for therapeutic use in New Zealand is slim to none.
RELATED ARTICLE: Meet New Zealand’s Most Influential Cannabis Activist
Abe Gray is a radio personality, researcher, and curator of New Zealand’s only cannabis museum. He’s skeptical, at best, that this will change the mind of the Minister of Health.

“The current approach of the NZ Ministry of Health tends to ignore the evidence from people who have successfully used various cannabis products.” He added, “My experience indicates that the real problem is not the lack of evidence, but the lack of resources to collect, assess and circulate that evidence in a transparent way and a lack of education of doctors and other medical experts on how and why cannabinoids work and how they can most effectively be used.”
RELATED ARTICLE: New Zealand Waits for Their Cannabis Tipping Point
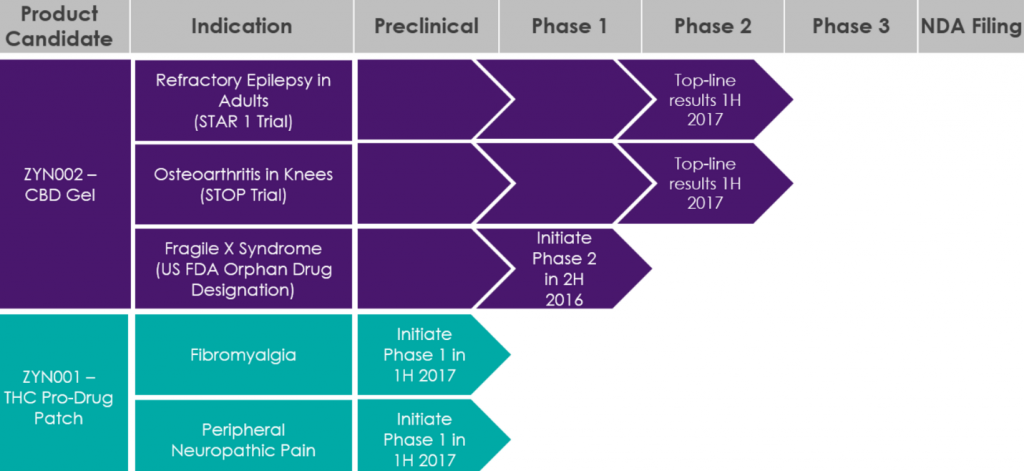
The latest announcement confirms the initiation of enrollment of the first patients into the STAR 2 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy) clinical trial.
The trial will allow patients who have completed the STAR 1 clinical trial to receive treatment with ZYN002 CBD gel for up to 52 weeks to get a better understanding of the long-term effects.
“STAR 2 is designed to provide additional tolerability and safety information for ZYN002 CBD gel for up to 52 weeks, which we believe will establish that ZYN002 CBD gel is well-tolerated over long-term use,” said Armando Anido, CEO of Zynerba.
The clinical trial details
The clinical trial is specifically for epilepsy patients with refractory focal seizures. The patients will receive 195 mg of CBD in ZYN002 4.2% gel every 12 hours. After two weeks, patients may have the option to reduce the dose to 97.5 mg of CBD.
The clear gel is designed to provide consistent, controlled drug delivery transdermally, twice a day. Applying the drug to the skin avoids first-pass liver metabolism, and potentially enables lower dosage levels of active pharmaceutical ingredients in the medicine, among other benefits.
The clinical trial is being conducted at the same 14 sites in Australia and New Zealand as the STAR 1 clinical trial.
The gel is the first and only synthetic CBD formulated as a patent-protected permeation-enhanced gel and is being studied in Fragile X syndrome (US DEA orphan drug designated) and osteoarthritis in the knees.
Medical marijuana in New Zealand
Very few Kiwis are allowed to use medical THC and CBD. “The free flow of information and education is hampered by the current law in New Zealand and the risk of prosecution of anyone who uses cannabis products, explained Grey, a lawyer by trade.
The exemption has been for the very small number who have successfully navigated the many hoops and hurdles to get the Minister’s approval. It’s a foreign concept to many in America, as more than half of the states have a medical or recreational program.

“With four more U.S. states legalising recreational cannabis use this month, the evidence is overwhelming that prohibition can no longer be legitimised with arguments about public safety,” said Grey.
Taking a note from America, Grey wonders how the fate of every patient in New Zealand who wants to use the plant medicinally must first go through Peter Dunne, the Minister of Health.
“…wouldn’t it just be easier to legalise or decriminalise cannabis and then all patients and their caregivers would have access to the full range of products and innovations in this space immediately, just like they do in the USA,” she said rhetorically.
Instead, Kiwis must suffer through the ‘draconian’ Ministerial approval process currently in place.
“If Peter Dunne and the Government would just…show a little common sense and compassion, needless deaths of great New Zealanders like Helen Kelly and Alex Renton could be avoided. Our current system is out of all proportion regarding risk and out of step with other progressive western countries.”
An international perspective
In America, CBDRx has the first U.S. Organic Hemp farm in Colorado from which they manufacture all their CBD products. CBDRx is involved in a clinical study in Brazil, providing 50mg capsules to the study.
Regarding the clinical trial on the CBD gel, Pamela Clum, botanist for CBDRx said, “Any country allowing clinical studies is welcomed and embraced. It’s a good thing all round because there are not enough clinical trials.”
For worldwide medical and patient communities, the clinical trial for this CBD treatment is positive on many levels. “It is a step forward for the many New Zealanders who are seeking alternatives to the drugs that are currently available,” said Grey.







The transnational criminal corporate cabal that uses nation/states as middle-management has an APB out on cannabis since it supports deprogramming and self-sufficiency.