There are two ways to reschedule a drug in America, through Congress or Executive action, and experts agree that Congressional action is the easiest and more realistic way to remove the Cannabis plant from its Schedule I status within the Controlled Substances Act.
The Drug Enforcement Agency (DEA) and Department of Justice (DOJ), in accordance with the federal U.S. government’s Controlled Substances Act, are charged with enforcing all penalties and distribution of drugs listed within the Act’s five category system. Where a substance is placed depends on its accepted medical use and the drug’s potential for dependency. Schedule I status is the most restricted group of the five, denying any medical use and associating the listed drug as having a high potential for abuse. Schedule I drugs include Heroin, LSD, MDMA, and marijuana. The term ‘marijuana’ includes both the cannabis plant (extracts and tinctures) as well as the tetrahydrocannabinol compound, more commonly referred to as THC.
Steph Sherer, the Executive Director for Americans for Safe Access, takes umbrage with the ‘no acceptable medical use’ clause and took a stand against this conventional wisdom along with dozens of other scientists and data analysis experts at the Americans for Safe Access Unity Conference in Washington, D.C. this past weekend.
“We are coming to a point in America where everyone knows someone who was medically helped by marijuana,” says Sherer.
The rescheduling of cannabis is a hot topic in Congress right now as the CARERS Act gains momentum with bipartisan support. The pro-cannabis legislation calls for the rescheduling of cannabis from I to a schedule II drug, which would lift the ban on medical marijuana research and it would give veterans access to medical marijuana. Current supporters of the bill include: Senators Kirsten Gillibrand (D-NY), Cory Booker (D-NJ), Rand Paul (R-KY), and most recently Senator Lindsey Graham (R-SC).
Senator Barbara Mikulski (D-MD) is another supporter, calling medical marijuana “an issue Congress can no longer ignore…for safe and legal access to this important medical therapy” in a pre-recorded video for the conference. Sen. Mikulski closed her message with a call to action in passing the CARERS Act with a rallying, “let’s just do it.”
The Lowes Madison Hotel conference room in Downtown Washington, D.C. was filled with the greatest minds when it comes to cannabis science. Most notable was Dr. Lumír Ondřej Hanuš, the Czech analytical chemist who, along with William Anthony Devane, first isolated and then described the structure of anandamide, an endogenous cannabinoid neurotransmitter.
Ethan Russo, medical director of PHYTECS gave a presentation on the history of cannabis. He started his remarks with this moment of levity, “sometimes you have to laugh to keep from crying, when it comes to the dark history of cannabis.”
The two major events that changed the course of the plant was the first Federal Bureau of Narcotics commissioner, Harry Anslinger’s Marihuana Tax Act of 1937 and President Nixon’s Shafer Commission.
Dr. Hanuš began his medical marijuana research in the 1970’s right around the formation of the Czech Republic. He says he was lucky to be able to work on cannabis in the Czech Republic and with a great professor when his work would have been condemned in countries like the U.S. His data-rich presentation and thick accent made it difficult at times to understand his every word but the accuracy of his work remain untouchable.
Dr. Hanuš spoke about being published with the world-renown cannabis researcher Professor Raphael Mechoulam, of Hebrew University in Israel. Mechoulam is responsible for the ground-breaking discoveries of the structures of Cannabidiol (CBD) and tetrahydrocannabinol (THC), the two main compounds found in cannabis. These discoveries in the early 1960 have set the foundation for what we know about cannabis today.
In 1992, Mechoulam, Hanuš, and Devane identified the brain’s first endogenous cannabinoid. They named it Anandamide (ANA) and Hanuš describes its function as “a molecular messenger which plays a role in pain, depression, appetite, memory and fertility.” It took time to isolate the cannabinoid, and a lot of trips to the butcher for animal brains, Hanuš quipped.
“Some of it is luck, said Hanuš, the THC from the plant and Anandamide together resulted in interesting, something worth exploring,” and he was right.
ANA and THC work together like two keys that unlock the CB1 or CB2 receptors, releasing the medicinal properties of the plant as shown in the following diagram:
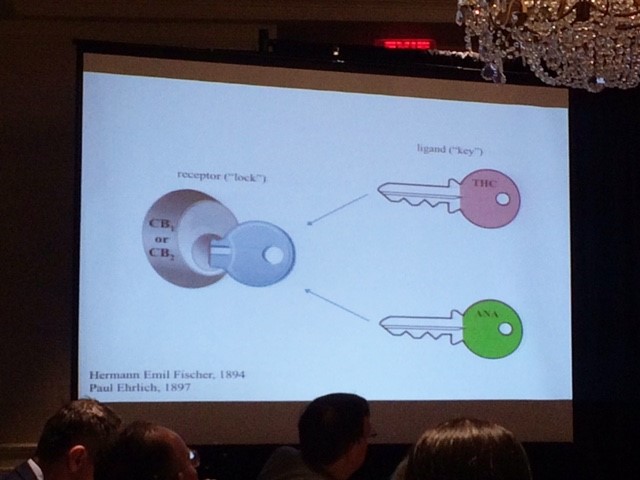
Hanuš’ findings show that appetite, blood pressure, memory, ‘almost everything’ is affected by cannabinoids. On the other hand, he warns that CBD and THC are not necessarily cure-all treatments. Cannabis is a ‘wonderful medicine’, he says, but does not cure every time, it doesn’t cure everyone, it won’t cure every stage of a disease, and it won’t stop every disease.
Imagine a world where you are given the news that you have illness X, then your doctor can prescribe you a strain of cannabis dependent on your body and the disease. Dr. Hanuš says if physicians and scientists work together, it can happen in the next ten years.
“It’s not one medicine – it’s a huge amount of medicines and we are all genetically different. Sometimes different strains or amounts for each person are needed and that’s why we must study it,” he concludes, “it’s not a game of cannabinoids but also other important compounds, probably flavonoids and others working together, the synergy between them is important to study chemically and medically.”
This proven system of your body’s natural receptors specifically designed for ANA and THC could be the key to trailblazing science in the medicinal field. Imagine a world where you are given the news that you have illness X, then your doctor can prescribe you a strain of cannabis dependent on your body and the disease. Dr. Hanuš says if physicians and scientists work together, it can happen in the next ten years.
“Right now Obama made it clear he won’t work on the effort this year which is unfortunate”, says Capecchi.
Cannabis research in America cannot go forward on a federal level as long as cannabis remains a Schedule I substance. As Grace Wallack, Senior researcher at the Brookings Institution explained at the Unity Conference, the hurdles currently facing medical marijuana are obtaining licenses to study schedule I drugs and that the only availability of medical marijuana is from the National Institute on Drug Abuse (NIDA) ‘monopoly’. The other barrier is cultural knowledge, stigmas and for many doctors, it’s not part of their training.

“There is potential here for a medical product and openings in the medical field to study it,” but Wallack’s research paper published by Brookings exposes the red tape keeping America’s researchers away from cannabis:
“Rescheduling would have substantive effects beyond changing perceptions. DEA, FDA, and state law all require levels of licensing and registration for conducting research with Schedule I drugs. Researchers hoping to obtain approval for research with marijuana (or any Schedule I drug for that matter) go through a multi-agency registration and review process. For any research with marijuana, researchers must undergo the FDA’s Investigational New Drug (IND) application, and NIH-funded projects must also undergo an additional, three-step NIH review. Researchers then obtain a DEA registration for possessing marijuana for research (this is also true of any Schedule I drug). Unlike other Schedule I drugs however, researchers then submit their proposal and request for study drugs to NIDA for review and to approve the supply of the drugs they need.14 Both the DEA-mandated NIDA monopoly on research marijuana and DEA registration represent hurdles to marijuana research that are not present for Schedule II drugs—or even other Schedule I drugs.”
Robert Capecchi, Director of Federal Policies for the Marijuana Policy Project, wasn’t at the conference but in an earlier interview for The Marijuana Times says Congress is the best bet for rescheduling.
Just take a look at this diagram from Wallack’s report for Brookings of what it would take for Congressional actions versus initiation by the President:
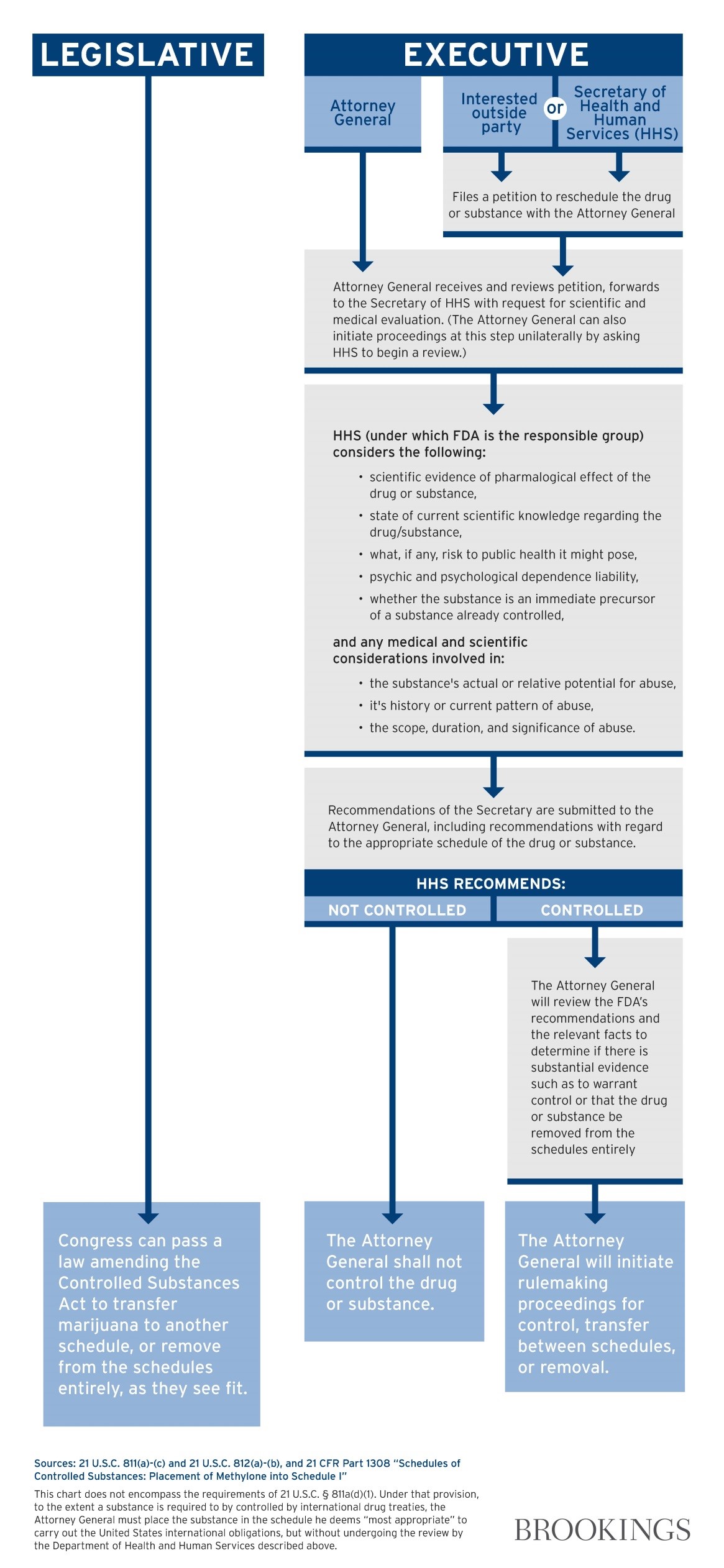
“Right now Obama made it clear he won’t work on the effort this year which is unfortunate”, says Capecchi.
The problem with an act by the President is the tremendous interagency cooperation and vigilance needed to get the job done. He admits it’s “easier” for Congress to just pass a bill, however, moving anything through Congress is a “tall order” as well. The way things are right now, Capecchi predicts cannabis will be rescheduled through an act of Congress and signed by President instead of executive action.
At the end of her remarks at the Americans for Safe Access Unity Conference, Wallack separated herself from her role at the Brookings Institution to give her true opinion on rescheduling. She thinks placing cannabis as a schedule II drug is better than descheduling it completely because states will become very defensive. in Wallack’s opinion , “schedule II won’t open the floodgates but it will open research on CBD for pain and other ailments and that’s what lawmakers want to hear.”
A representative of Senator Kirsten Gillibrand took the floor at the end of the conference for a short PSA on how to pitch the bill to lawmakers. Lawmakers will tell you they are waiting for the research to come in before supporting the CARERS Act – remind them, there is no research allowed without this bill.
The science discovered abroad and shared at the Unity Conference has not yet made its way into mainstream America but that’s changing with new cannabis-derived treatments like Epidiolex to lessen seizures in epilepsy patients and Sativex for treatment of spasticity from Multiple Sclerosis. Currently, neither treatment is approved in the U.S.
RELATED ARTICLE: America is the Next Stop for Cannabis Epilepsy Medication, says British Biopharmaceutical Company
According to the GW Pharmaceuticals, Sativex is undergoing Phase III trials in the U.S. for cancer pain. Epidolex has made it to third round clinical studies, prompting GW Pharmaceuticals to request a pre-NDA meeting with the FDA to discuss proposed regulatory submission. Statistically significant results from the trials could translate to legal distribution in the U.S. under The Orphan Drug Act (ODA).
The Marijuana Policy Project’s Robert Capecchi remains cautiously optimistic.
“There are two marijuana related de/re-scheduling petitions under consideration. One of them is for marijuana as a whole, the other is CBD specific. If anything, this study might speed up the timeline of the CBD de/rescheduling petition process, but I don’t see it having much impact on the timeline for de/rescheduling marijuana as a whole.”
As Russo reminded everyone at the Unity Conference, differential scheduling is possible.
“Even if specific preparation attains FDA approval, herbal cannabis will remain in Schedule I,” stated Russo.







EPIPHANY : It should be 100% legal for every adult & child to ingest fresh raw NON psycho-active marijuana with large amounts of all cannabinoids (including THCa) . So that our bodies natural endocannabinoid system can fight illness & disease (including cancers) . Denying people this right is a Heinous Crime . Excessive recreational alcohol enables Random Violence , Suicide , Vehicular Homicide , Domestic Violence , Child Abuse , Date Rape & The Use of Harder Drugs . Recreational psycho-active marijuana is 100% SAFER than alcohol . Medical marijuana is waiting to become ill risking death while Recreational marijuana is preventing illness from occurring .